Fuel Cells

SOFC convert chemical energy in the fuel directly into electricity without burning it, enabling high-ecient electricity and contribute to the CO2 reduction.It can use a wide variety of fuels, including natural gas, biomass and hydrogen.
Principles of Power Generation
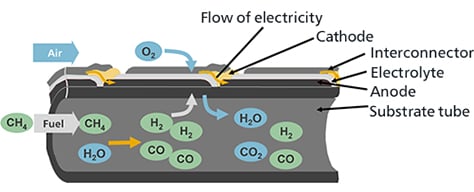
The SOFC generates power at between 700°C and 1000°C by being supplied fuel gas (hydrogen, carbon monoxide, etc.) to the fuel electrodes and air (oxygen) to the air electrodes.
Methane (CH4), the main ingredient of the fuel gases inserted into the cell stack, and water vapor (H2O), which is contained in the exhaust fuel that is recirculated, become hydrogen (H2) and carbon monoxide (CO) inside the cell stack due to the internal reforming reaction that is a characteristics of SOFC.
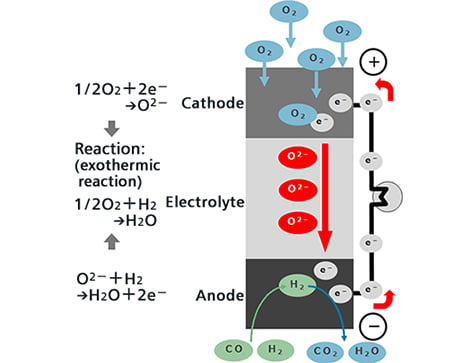
Oxygen ions (O2-) that move from the air electrode side to within the electrolyte react with the hydrogen (H2) and carbon monoxide (CO) of the fuel at the interface between the fuel electrodes and electrolyte, emitting electrons (e-) while simultaneously generating water vapor (H2O) or carbon dioxide (CO2).
Meanwhile, after the electrons emitted by the oxygen ions have performed electric work through the outer electric circuit, they move to the air electrodes.
At the interface between the air electrodes and electrolyte, oxygen in the air (O2) reacts with the electrons that have moved over to produce oxygen ions, and these oxygen ions are captured in the electrolyte and move to the fuel electrode side.
In terms of overall power generating reaction, hydrogen or carbon monoxide reacts with oxygen to generate water or carbon dioxide, and electricity flows with the resulting electrons move through the outer circuit.
The air electrode is the cathode, and the fuel electrode is the anode.
Products
- GTCC
- Steam Power
- IGCC
- Geothermal
-
Gas Turbines
- Product Lineup
- Comparative Performance
-
Technical Information
- Gas Turbines for Mechanical Drive Applications
- Cutting-Edge Elemental Technology Producing 1600°C Class J Gas Turbines
- Development of High-Efficiency Gas Turbine Applying 1600°C Class J Technology
- Combustor Technologies Supporting Stable Operation
- Overview and Verification Status of T-Point 2 Demonstration Facility
- Comprehensive Efforts from Development to Manufacturing
- Summary of Orders
- Development History
- Product Selection Assistant (Middle & small Class)
- Aero-derivative Gas Turbines
- Steam Turbines
- Boilers
- Air Quality Control Systems (AQCS)
- Generators
-
Control Systems
- What is DIASYS?
- DIASYS Netmation
-
DIASYS Optional Products
- IR-S Infrared Flame Detector
- Net IR-S Infrared Flame Detector
- Rail Mounting Net IR-S
- Boiler Tube Leak Detector
- Shaft Vibration Analyzer
- Simulator
- Advanced Combustion Pressure Fluctuation Monitoring System (A-CPFM) / Combustion Pressure Fluctuation Monitoring System (CPFM)
- Multi-Coal Fired Boiler Optimum Control
- FXtoLS Adapter
- Fuel Cells
- Additive Manufacturing
- Catalogue
- HIACS Series
- Technical Report